Insulating glass inflation control and Low-E glass coating detection.

Abstract: Modern insulating glass technology, namely low-emissivity film coating and inert gas filling technology, can fully improve the thermal insulation function of doors and windows. These advanced ingredients or materials are difficult to detect with the naked eye, especially when it is almost impossible to detect what kind of inert gas they are and how much of the inert gas they contain. In addition, it is difficult to determine whether there is a low-emissivity coating on the insulating glass, the location of the coating, and the thickness of each layer of glass.
Both glass deep processing manufacturers and their customers should be able to test the insulating glass they produce or purchase to ensure that these products meet their expectations and needs.
Currently, there are several ways to detect the gas content within inflated insulating glass and the structure of the insulating glass. Likewise, instruments are being developed to detect the presence and location of Low-E coatings on insulating glass units, so that the overall quality of the insulating glass units can be assessed. This article is intended to give readers a basic understanding of several different methods available on the market.
Keywords and Terms: Low-E glass coating, inert gas, argon, gas chamber layer, U-value, gas content, gas retention
1. The Overview
No matter where you are in the world today, the demand for high-quality insulating glass is growing day by day. Many countries have made international or local commitments to reduce carbon dioxide emissions. Energy demand is growing, driven by the world's growing population and economic growth. The basic goals of energy production and environmental impact are to reduce the rate of global warming and to produce energy safely. At the beginning of the 21st century, the increase in energy consumption has shown the fastest growth rate of 3% on average per year. This phenomenon comes from rapid economic growth, such as in India and China. Energy production on a global scale is based on fossil materials. Carbon dioxide emissions are considered the main cause of the so-called greenhouse effect, and energy is its largest source. Carbon emissions are expected to fall to their average annual levels, especially for the industrial sector, the largest source, which is set to be very high. Reaching the goals in the construction sector will require changes to established practices and regulations. In the construction sector, the goals are divided into two stages, firstly to reduce the energy use of buildings, and secondly to replace the reduced energy demand with renewable energy. As you can tell from multiple international construction projects, investing in energy-efficient buildings is a wise choice. Although the cost of low-energy buildings is 2 to 5 percent higher than that of ordinary buildings, the long-term cost is still low because energy costs are lower. To reduce carbon emissions from building construction, government agencies have introduced formal laws and regulations to control the energy efficiency of insulation and building materials. When it comes to a building's insulation, glass is often the weakest link, so improving a building's overall energy efficiency is a powerful driver for improving the quality of insulating glass. In Finland, electricity and heating in buildings account for approximately 34% of total energy consumption. These regulations also set requirements for doors, windows and the glass used to make them, including the materials and structure used for insulating glass.
When studying the energy-saving effects of insulated glass, observers will focus on its insulating effect and the emissivity of the glass. This article will focus on the glass parts used in insulating glass. Unless otherwise defined, the U-value referred to in this article is the thermal conductivity coefficient. It describes the heat transfer from the air on one side of a unit area of a glass assembly to the air on the other side under standard conditions per unit time. That is, U = 1/R = Qa /dT(W/m2K) (unit: watts per square meter per Kelvin temperature). It is also known as the derivative of the R-value, which is the thermal resistance coefficient. The lower the U-value, the better the thermal insulation performance of the glass.
Table 1: Effect of glass part on window performance
| Factors | Influence of Insulation Effect |
| Glass thickness | Small |
| Distance between glass pieces | Medium |
| Number of glass pieces | Large |
| Inert gas | Medium |
| Different low emissivity coated glass | Large |
2. Low-emissivity coated insulating glass
The term "selective radiation" or "Low-E" glass refers to glass with one or more transparent films of metal oxides. Selective radiation means that the transmission and reflection of radiation depend on the wavelength of the radiation. The significance of low-emissivity coating is to reduce the heat radiation between the glass sheets of insulating glass to improve its thermal insulation performance.
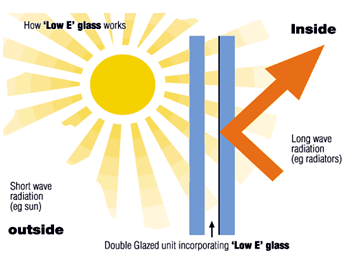
Heat transfer is achieved through gas flow, conduction, and radiation. Radiation is divided into longwave and shortwave radiation. Short-wave radiation such as visible sunlight, part of the long-wave thermal radiation incident on the glass will be filtered by the Low-E coating. This improves the insulation and overall thermal performance of the glass. Likewise, frost formation on interior surfaces is reduced due to low-e coatings. Frost is caused by differences in temperature and humidity between different pieces of glass. If the surface temperature of the insulating glass is lower than the temperature of the air inside it, it will also cause cold air to flow around the window. However, if the insulating glass used has good thermal insulation properties, there will be no problem because the temperature of the hollow surface will be relatively high. Some will not produce such a big temperature difference with the air inside.
For a standard window, nearly half of the heat loss is due to radiation between the glass sheets, while the other half is due to heat conduction and convection of inert gases.
Table 2: Examples of U-values for different window structures: windows used in developed countries and regions in Northern Europe or North America since 1978
| Year | Type | Structure | U-value of the glass (W/m2K) |
| 1978 | 1+1+1 | 4+50+4+50+4 | 1.8 |
| 2003 | 1+2 | 1+100+2g (4-12-E4) | 1.2 |
| 2010 | 1+2 | 4K+100+2g (4-15Ar-E4) | 0.8 |
| 2012 | 2+2 | 4-18Ar-E4+50+4-18Ar-E4 | 0.5 |
Figure 1 The effect of Low-E coating in insulating glass
3. The inert gas for insulating glass
3.1 Why should the two pieces of insulating glass be inflated?
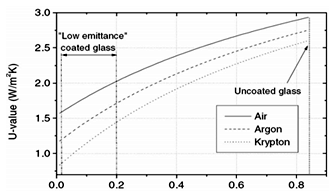
Rare gases such as argon, krypton, and even xenon are generally used as inert gases to fill insulating glass because their heat transmission and conduction are much smaller than standard air. This is why inert gases are used as filling gases. The biggest improvements can be achieved by plating low-emissivity coatings and filling them with argon gas. Argon is the most widely used inert gas because it is cheap and easy to extract. Argon is non-toxic and inactive and has about 30% lower thermal conductivity than air (0,0179 W/mk vs. 0,0262 W/mK). It is extracted from the air through the process of making liquid nitrogen and oxygen. As an inert gas, argon also protects other valuable materials within the insulating glass. More expensive options than argon are krypton and xenon, which are more stable and less reactive than argon. Sulfur hexafluoride has also been used for insulation due to its acoustic properties, but it is now considered to pollute the environment and is rarely used anymore.
Heat losses from insulating glass can also be reduced by optimizing the width of the spacers between the glass panes. Commonly used spacer widths are 16mm or 12mm, and spacer bars exceeding 18mm are rarely used. Convection of gases can be reduced by selecting an optimal inert gas with a density higher than that of air. Spacer bars are typically made from a thermally conductive material, such as aluminum or steel, creating a linear thermal bridge. Advanced spacer materials can reduce heat loss through their thermal conductivity, allowing the inside of the insulating glass to maintain the same temperature as the heated building when it is cold.
There are different standards indirectly or directly through the requirements for the U-value of the building, and according to these standards, the requirements for the argon gas filling rate are also different. Different standards may vary depending on the market region, but most of them set high requirements for production, that is, requiring an aeration content of 90% and an annual leakage rate of less than 1%.
Figure 2 The relationship between the U value of the third side of the glass and the emissivity under different gases and different contents, when the spacer width is 12 mm
3.2 Problems with insulating glass inflation and gas retention
Several potential problems can occur during the inflating process of insulating glass. Inflation can be performed by automatic box inflation or manual inflation, but both have their own risks and quality control issues. When using an automatic inflation line, the operator needs to verify that both the machinery and the inflation process are working properly. To solve this problem, some insulating glass production line manufacturers will use the physical properties of inert gas to float upward, inflate upwards, and install gas detection sensors in the combined boxes to display the inflation process through the operation panel. Because noble gases are invisible and odorless, they are difficult to detect without the right tools. Manual inflation can also be prone to leakage due to human error during operation, such as when filling the inflation hole or sealing the second sealant. In addition, inflation may result in turbulence or problems with the gas not being evenly distributed. Although the inflation process may be successful, problems can still occur. Inert gases may slowly leak out due to poor materials or workmanship used in the production process. Therefore, the gas content should be checked several times during the production process to ensure that the entire production stage is operating as it was set up.
4. Detection and detection of inert gas content and low-radiation coating in insulating glass
4.1 Detection of low-radiation coatings
The coatings of selective radiation glass are generally metal and conductive coatings. In addition, coated glass will reflect more light than white glass, which can be used to detect whether the glass has a low-emissivity coating.
For capacitive detectors, alternating current is conducted across the glass surface through electrodes. Detectors can be designed for low-e coating detection or moisture detection. This type of detector will be able to detect the strength of the current. A piece of selectively radiating glass can produce a stronger current than plain glass. This method is used when detecting whether low-emissivity coated glass has a low-emissivity coating. However, it cannot be used for low-e coating inspection of multi-layer glass structures.
Another way to detect whether there is a low-e coating on the glass surface is to conduct direct current through one electrode to another electrode and then conduct it on the glass surface. At this time, the coated surface will conduct the current and the uncoated side will behave as an insulator, because the glass is not a conductor but an insulator. Operators using this solution need to take measurements from a known coating surface.
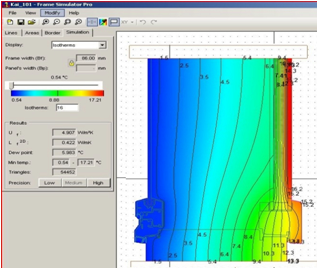
Optical measuring instruments are also used to detect low-emissivity coatings on glass and detect glass spacer thickness. These optical measuring instruments allow the operator to simultaneously detect the thickness of the glass intervals when testing the thickness of each glass sheet of insulating glass. An example of the instrument is shown in Figure 2. This technology is based on a laser beam that is emitted at a 45-degree angle and is then reflected to the instrument sensor. The thickness of the glass sheet and spacer is determined by the distance from the reflected laser beam to the sensor. This technique also requires prior knowledge of the refractive parameters of glass and air.
There are many such instruments on the market for detecting the thickness of Low-E coatings and spacers. Its range of features and prices vary widely depending on the end user's needs. For some instruments, the operator must base summaries on reflections in certain ranges. Similarly, some more complex but user-friendly instruments can tell users the test results of insulating glass in a graphical way. Such an instrument can inspect the entire configuration, checking that the Low-E coatings are in the correct position and that the glass panes and spacer widths are within allowed tolerances. Conclusions regarding potential excess pressure are also measurable with appropriate instrumentation.
Figure 3 An optical inspection instrument for measuring glass and spacer thickness
4.2 Why is it important to verify the inert gas content in insulating glass?
Unlike others, many factors that affect the gas permeability of insulating glass are important, such as the elasticity and firmness of the sealant, the aging of the sealant, the desiccant material, the thickness and width of the sealant coating, the penetration Spreading speed of spacers and sealants, etc. When it comes to production processes, design, workmanship, and inflation technology, all should be controlled as much as possible.
The performance of insulating glass is affected on the one hand by the diffusion of moisture into the insulating glass unit and on the other hand by the diffusithe beginning of the application of inert gas, the glass industry has been studying methods for detecting inert gas content. Different oxygen analysis methods have been used by various industries for quite some time. The influx of oxygen into industrial gases is tested every day around the world, as are gases in the medical industry and diving.
In this regard, the analysis methods of gas content in insulating glass can be divided into three types: physical methods, chemical methods, and sampling methods. This chapter will examine these three different methods.
4.3.1 Physical detection methods to detect inert gas content
The physical method is based on the deterperties of certain elements, gases being unique to each element. Such properties refer to things like thermal conductivity, speed of sound, weight of molecules, size of molecules, radiation absorption coefficient or radiation waves emitted by plasma, etc.
The absorption of electromagnetic radiation waves by gases and the amount of radiation depends on the gas content in the sample. For example, gases absorb specific wavelengths of infrared radiation. This method is applied to the detection of carbon dioxide in fire alarm systems. However, it is difficult to detect noble gas content using this method because the radiation wave must pass through at least two layers of glass, which already filters out the relevant wavelengths.
Radioactive radiation wave absorption is used in fire alarm systems to detect fire gases. Glass absorbs alpha radiation waves, but it cannot detect noble gases, while beta and ϒ radiation waves are unsafe for use in mobile equipment under production conditions.
The study of the spectrum of radiation waves emitted by ionized gases was proposed in Finland at the end of the 20th century. The technology is based on activating gas atoms of a noble gas so that they emit a beam of light through the glass that can be analyzed. Each gas has its unique spectrum, and these spectra can be identified. The gas content in the sample will affect the intensity of the radiation wave, which makes it possible to detect the gas content in the insulating glass.
This technology can accurately and repeatedly detect the argon and krypton gas content in insulating glass and is a non-destructive test. The operator must know whether the gas he/she is detecting is argon or krypton. Because this method is based on the activation of gas atoms by high-voltage discharges, it has some limitations. The electric spark must penetrate the air cavity layer so that the measurement becomesfeasible. Sparks cannot jump over low-emissivity coated glass or thick laminated films, as this would make measurements difficult. The background light is also sensitive to the impact of measurement results, so when measuring, you should always try to conduct it under standard conditions. It is recommended that the background be supported by a matching bracket covered with black cloth, or a similar background. To get around these limitations, manufacturers usually prepare testable samples separately for testing. For example: when making a three-glass two-chamber hollow, they will put a glass plate coated with a low-emissivity film in the middle, so that The inert gas content of the two chambers can be measured separately.
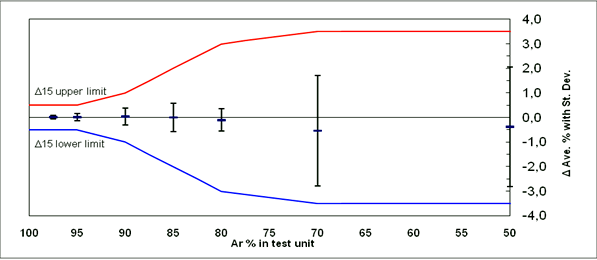
Table 3 Gasglass Technology Accuracy and Repeatability
| Argon content in the tested sample | Measured average | Average deviation | Standard deviation |
| 97.5 | 97.5 | 0 | 0.1 |
| 94.9 | 94.9 | 0 | 0.1 |
| 90.0 | 90.0 | 0 | 0.3 |
| 85.1 | 85.1 | 0 | 0.6 |
| 80.2 | 80.1 | -0.1 | 0.5 |
| 70.9 | 70.4 | -0.5 | 2.2 |
| 50.1 | 49.7 | -0.4 | 2.4 |
Figure 4 Gasglass Technology Accuracy and Repeatability
Table 3 shows the accuracy and repeatability of the third generation of Gasglass instruments. As can be seen from the report, the accuracy limits for each aeration content are given in terms of standard deviation, and the mean value of the measurements here refers to the repeatability of the device. As can be seen, the accuracy and repeatability of this technique are based on the level of inflation. For example, when the inflation reaches 94.9%, the accuracy is very high and the standard deviation is very small. When the inflation rate reaches 70.9%, the measured average value shifts down by 0.5%, and the sum of the standard deviations is 2.2. However, this can be fully used for inert gas content detection. From the relevant figures, we can see that each instrument has its unique calibration curve. The manufacturer of Gasglass instruments recommends that users calibrate their instruments annually. The calibration process of Gasglass is relatively complex. It requires 8 different calibration points and must be calibrated by the manufacturer or a certified service provider. To date, the manufacturer has three service centers worldwide, in the United States, China, and Finland. Each calibration point is calibrated using certified standard gases. Spectroscopy based on instantaneous discharge or plasma emission techniques is now widely used in the glass industry around the world.
Physical methods for the analysis of other noble gases have also been studied. These methods are based on different technologies such as tunable diode lasers, Raman spectroscopes, changes in the speed of sound in materials, or changes in thermal conductivity in noble gases. Regardless of the method used, when it comes to analyzing the gas content of insulating glass, all methods have their difficulties or limitations that, as of now, there is no way to solve so that they can be applied in the market. Instruments based on breakthrough voltage change technology are now on the market but not many, because many factors affect their measurement results.
4.3.2 Chemical methods for detecting inert gas content
Noble gases are used as inert gases that form compounds with other elements only under special conditions. The inert gas content can be analyzed by analyzing the gas component content of the air remaining in the air cavity layer after inflation. In this case, the method does not verify the noble gas itself but rather identifies other gas components of the air remaining in the gas chamber layer.
Some materials may change their color due to changes in the oxygen content of the gas mixture. This is like sticking a piece of paper on it that will change color over time. This method can be used, for example, to check the difference between insulated glass samples before and after climate cycling.
4.3.3 Sampling Analysis Method
The sampling analysis method provides users with a reliable method to determine the type and content of inert gases. There are many techniques for analyzing gases using sampling methods, which are also used by production companies in the gas industry. Taking samples from hollow glass will always destroy its integrity. Usually, it is necessary to punch holes in the spacer, and the sample can be taken out with a syringe for analysis or directly inserted with a measuring instrument for analysis. Gas samples require different volumes, from µL to mL, depending on the technology used. The gas chromatograph requires an extremely small amount of gas sample, so measurements can be repeated. Although the manufacturer describes how to properly seal the hole drilled for sampling, gas is still prone to leaking. If an oxygen analyzer is used to sample, the gas sample volume will be relatively large, making it impossible to perform repeated measurements.
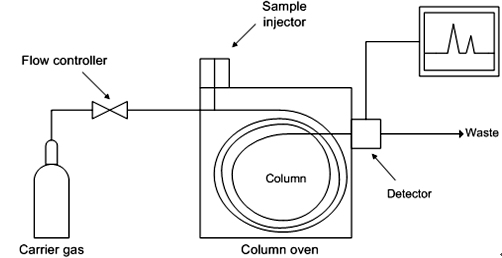
4.3.3.1 Gas chromatography analyzer
Gas chromatographs with different types of sensors have been on the market for some time and have always been considered to be highly accurate and reliable. The amount of gas sample that needs to be extracted is also very small, about 25µL, allowing the operator to repeat the measurement many times. The gas chromatograph uses gas chromatography, whose components are gases, and is suitable for qualitative and quantitative analysis.
Figure 5 Gas chromatograph operating principle diagram
Chromatographic separation is the separation of chemical components of a complex system. A chromatograph uses a fluid phase passing through a narrow tube, called a chromatography column. The components of the sample move through the column at different rates based on their unique chemical and physical properties, where the known phase interacts with the stationary phase. As chemical components leave the column, they are detected by various electronic detectors. The stationary phase in the column is supposed to separate the different components of the gas sample being analyzed. Likewise, the temperature within the column and the flow rate of the gas sample are controllable. Several different types of detectors are used in gas chromatographs. The most common types are flame ionization detectors and thermal conductivity detectors. The gas chromatograph can also be connected to a mass spectrometer which will operate as a detector.According to the author's experience, gas chromatography is often used as a traceability instrument in laboratories in the glass industry around the world. This approach would be too complex, time-consuming, and expensive to implement in a production plant.
4.3.3.2 Oxygen analyzer
A wide variety of oxygen analyses can be found on the market. In addition to the glass industry, oxygen analyzers are widely used in the medical industry and are also used in diving equipment to detect oxygen content. They can detect the oxygen content in a gas sample, allowing operators to determine the content of the gas being measured. This determination is based on the assumption of various gas content in the air. For example, air contains approximately 78% nitrogen, 21% oxygen, 0.94% argon, and other gases. The oxygen analyzer uses these data to calculate the oxygen content in the gas sample and obtain the total amount of air. It is expected that all that will be left in the gas sample at the end is argon (or krypton).
Oxygen analyzers generally have many types of sensors that can be used. Common ones are electrochemical batteries and paramagnetic batteries. Electrochemical cells, also known as fuel cells, measure the trace percentage of oxygen in a gas or gas mixture. The gas sample being analyzed enters the sensor through a gas-permeable membrane. The oxygen in the gas sample is dissolved in the electrolyte after contact with the anode and cathode in the sensor. The flow of electrons and current generated from the cathode to the anode is proportional to the oxygen in the gas sample. These electrochemical cells wear out over time because the lead anodes used in the sensors are fragile in the presence of high oxygen levels. Once the lead anode is oxidized, the battery no longer produces output and is useless unless replaced with a new lead anode. The operator will only notice this if the instrument can no longer be calibrated or if calibration takes an extremely long time. Here, take the Sensoline handheld oxygen analyzer as an example. It uses an electrochemical cell and is easy to use because it has an integrated pump inside, so the operator does not need to manually extract and inject gas samples because the measurement Results may be dependent on the gas sample flow rate. The operator can take some air as a gas sample and then calibrate the instrument with a simple button operation.
Operators should take care when doing calibrations because analyzers like this can drift over time. The manufacturer states that this technology allows an accuracy of 0.1% when calculating argon content from measured oxygen.
Paramagnetic batteries take advantage of the fact that oxygen has a higher volume magnetic susceptibility than ordinary gases. A paramagnetic sensor is combined with two glass spheres containing a reference gas, usually nitrogen, mounted on a suspended rotating device. This device, commonly known as a dumbbell, contains a powerful magnetic field. When a gas sample containing oxygen passes through the process and enters this sensor, the oxygen molecules are attracted to the stronger of the two magnetic fields. This force couple causes displacement within the dumbbell and then rotation. There is an optical system inside that is used to measure the rotation angle of the dumbbell. The function of the reverse current is to restore the dumbbell to its original position. That current is proportional to the oxygen partial pressure and can be converted into readable oxygen so the operator knows the oxygen content inside.
Paramagnetic sensors are generally considered to be durable. Paramagnetic sensors are very sensitive to vibration, placement, and application, and are generally of the "standalone" type, which means that in addition to the analyzer itself, it must be in a stable environment, including the injected gas sample and objects. The magnetic susceptibility exhibited by other gases may produce relatively large measurement errors, and manufacturers should provide detailed information on such gases. Depending on the requirements of the manufacturer, the operator may have to select the measuring range from the switch. At the beginning of the operation, the operator should analyze a sample of air to ensure that the instrument displays the correct value of 20.9%, as well as pure argon without any air present. As an oxygen analyzer with a paramagnetic sensor, in addition to Helantec's Helox KVSN-F, the German company KIWA zemlabor GmbH can also provide instruments with more or less approximate accuracy than the previously exemplified Sensoline oxygen analysis.
There are also oxygen analyzers on the market with other types of sensors, such as thermal conductivity sensors, zirconia sensors, ambient temperature sensors polarographic sensors, or a combination of many sensors.
5. Future research
As we said, due to global requirements for energy conservation and building energy efficiency, government departments and ordinary people are paying more and more attention to window energy-saving solutions. This also makes the insulating glass solution more closely linked to energy efficiency. We can see from the history of many markets that the requirements for the U-value of insulating glass have tightened, and in the future, expectations for this area will be even higher. This is achieved through high-quality materials, workmanship, and quality control.
Because the main gas analysis methods have their limitations, the industry needs to develop a new detection method that does not require glass structure and does not destroy the glass. As far as the author knows, the only Chinese equipment assembly manufacturer that can achieve insulating glass online inflation without damaging gas analysis is LIJIANG Glass. The company has installed a Gasglass device on its insulating glass production line to analyze argon gas.
Installing testing devices on the production line allows manufacturers to test every product produced and ensure the normal operation of the inflation process. This will also be a marketing advantage for manufacturing companies because they can tell customers that each of their products is tested during the production process and is randomly inspected before shipment.
6. Conclusion
The purpose of this article is to let readers know what technology can be used to detect the low-e coating on insulating glass and the content of inert gases in it.
We describe the main existing technologies for detecting the presence and location of Low-E coatings. Many different types of products can perform this task. Users need to set a feature requirement and product quality for themselves.
Through our introduction to different analysis methods for inert gases, we hope that readers will be able to understand the different detection methods that can be used. The most commonly used methods in the glass industry are mainly sampling analyses completed in the laboratory, such as gas chromatographs and oxygen analyzers with different sensors. In addition to sampling analysis, analyzers based on plasma emission spectrometry technology are also commonly used in the industry.
All methods have weaknesses in the detection process. Gas chromatography is widely regarded as the best solution. It requires very expensive machinery, fragile sensors, and very experienced users. This technology is mostly used in research laboratories in the glass industry. Oxygen analyzers are also widely used in the industry, but they also require sampling. The plasma emission spectrometry tester is also widely used in the industry and is the only non-destructive inert gas detection equipment. The weakness of this technology is that it cannot penetrate low-emissivity coated glass or thick laminated films, and the manufacturer needs to prepare some additional glass that can be used for testing. All detection methods can achieve the required accuracy. For reliable testing, operators should be familiar with the technology they are using to ensure that the results are trustworthy. The sampling test method is not repeatable, because the inert gas in the sample will have relatively large composition changes after each test. The only reproducible sampling test possible is gas chromatography with µL sample sizes. Non-destructive plasma emission spectrometry allows for repeatable testing. This also allows the operator to pay attention to the leakage rate of inert gas within the insulating glass and at the same time check whether the inflator is adjusted to optimal working condition.
For more information about insulating glass processing equipment and glass processing machinery, please click here to learn more.