Application and quality control of inert gases in insulating glass.

As energy-saving policies in various countries around the world are increasingly implemented, building energy-saving requirements are gradually increasing. Architectural glass has also seen a trend of upgrading to high-performance insulating glass. In addition to the low-emissivity coated insulating glass that is constantly being promoted, there is a new type of insulating glass filled with inert gases such as argon and helium in the middle layer. Compared with air, inert gas has a higher density and lower thermal conductivity, so it can slow down the heat convection in the middle layer and reduce the thermal conductivity of the gas, thus reducing the heat transfer coefficient of the insulating glass and helping to improve the thermal insulation performance and performance of the insulating glass.
Filling the middle layer with inert gas is beneficial to improving the thermal insulation performance of insulating glass, but the type of inert gas filled, gas concentration, concentration retention rate, etc. all have an impact on the degree of improvement in thermal insulation performance. LIJIANG Glass researches the application and quality control of inert gases in insulating glass from aspects such as the performance comparison of different inert gases, the relationship between gas concentration and insulation performance, and the testing of inert gas concentration and concentration retention rates.
1. The application of inert gases
Inert gases include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). The inert gases are colorless, odorless, non-toxic, gaseous single atoms. Molecules are in group zero of the periodic table. The outer electrons have reached saturation and their activity is extremely small.
1.1 Comparison of performance of different types of gases
Inert gases used for insulating glass are argon, krypton, and xenon. Their common characteristics are stable performance, inertness, higher density than air, and lower thermal conductivity. The densities of these three inert gases at the temperature of 0°C and the pressure of 101.325 kPa are 1.78 kg/m3, 2.86 kg/m3, and 4.56 kg/m3 respectively (the density of air under the same conditions is 1.29kg/m3), and the thermal conductivity coefficients are 0.0163 W /(m·K) respectively. 0.0087 W/(m·K), 0.0052 W/(m·K) (under the same conditions, the thermal conductivity of air is 0.0241 W/(m·K).
Of the 3 gases, argon is the most abundant in the air. Argon, which accounts for approximately 0.93% of air by volume, is the most widely used and one of the cheapest inert gases on the market. Argon-filled insulating glass is UV-resistant without affecting indoor light. The content of krypton in the air is 1.14×10-4%. Its stability and reactivity are similar to argon. Its thermal efficiency is 1/3 higher than that of argon, but it is more expensive. Xenon is the least abundant of the five rare gases in the air, with a content of only 0.09×10-4%. The stability and reactivity of xenon are also similar to argon, and its thermal efficiency is 1/2 higher than that of argon. However, xenon in its natural state is very rare and its purification price is high.
A comparison of the physical properties of the three gases relative to the air, as well as their effects on the heat transfer coefficient of insulating glass with the same configuration under the same aeration content, are shown in Table 1.
Comparison of performance of different gases
| Gas type | Content in the air / % | Densities / kg*m-3 | Thermal conductivity / W (m·K)-1 | Heat transfer coefficient / W (m·K)-1 (6mm white glass + 12mm white glass) Insulating glass |
| Air | 100 | 1.29 | 0.0241 | 2.667 |
| Argon | 0.93 | 1.78 | 0.0163 | 2.508 |
| Krypton gas | 1.14×10-4 | 2.86 | 0.0087 | 2.454 |
| Xenon gas | 0.09×10-4 | 4.56 | 0.0052 | 2.420 |
Illustrate: 1. Content in air refers to volume percentage. 2. Density and thermal conductivity refer to the conditions at a temperature of 0°C and an atmospheric pressure of 101.325KPa. 3. The heat transfer coefficient value is calculated by computer software assuming that the 12mm middle layer is filled with 100% gas content. | ||||
The following conclusions can be drawn from the above comparison:
(1) Argon, krypton, and xenon are denser than air and have lower thermal conductivity than air, which is beneficial to greatly improving the heat transfer performance of insulating glass.
(2) Krypton gas and xenon gas have 1/3 and 1/2 higher thermal efficiency respectively than argon gas. That is, to achieve the same heat transfer coefficient requirements under the same gas content, the insulating glass intermediate layer filled with krypton gas and xenon gas can only be It is 2/3 to 1/2 of the thickness of the argon-filled insulating glass intermediate layer.
(3) When the middle layer of the insulating glass is filled with argon gas, the heat transfer coefficient is 5.96% lower than when the middle layer is air. The effect of filling krypton gas and xenon gas is more obvious. The heat transfer coefficient is 7.99 lower than when the middle layer is 9.26%.
The above three inert gases can significantly improve the heat transfer performance of insulating glass. Among them, argon gas has a high content in the air and has a low inflation cost. It has become the most widely used gas for insulating glass inflation due to its high-cost performance; krypton gas The extraction cost is relatively more expensive than argon, and it is not widely used in the architectural glass industry. It is only used when the thickness of the intermediate layer of insulating glass is small but the heat transfer performance is high. The content of xenon in its natural state is very rare, and the purification price is very low. It is very high, so even though it helps the heat transfer performance of insulating glass better than argon and krypton, it is rarely used in the production of insulating glass.
1.2 Relationship between gas concentration and Thermal insulation performance
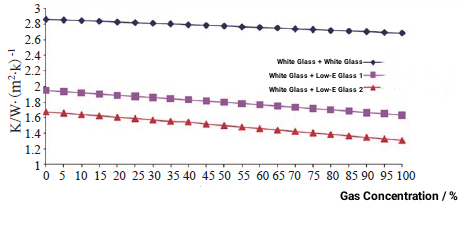
From the above discussion, it can be seen that argon has become the most widely used gas for insulating glass due to its outstanding cost-effectiveness advantages. The following will discuss the impact of the gas concentration on the performance of the insulating glass by comparing the heat transfer coefficients of three different configurations of insulating glass with different contents of argon gas. Different configurations of insulating glass are (4 mm white glass + 12 mm gas layer + 4 mm white glass), (4 mm online Low-E + 12 mm gas layer + 4 mm white glass) and (4 mm offline Low-E + 12 mm gas layer + 4 mm white glass). The heat transfer coefficient is calculated for each type of glass when the argon gas concentration is increased from 0 to 100% and every 5%. The results are shown in Figure 1.
Figure 1 Effect of gas concentration on heat transfer coefficient of insulating glass
From the above results we can get:
(1) The higher the argon concentration, the smaller the heat transfer coefficient of the insulating glass and the better the thermal insulation performance.
(2) Under the same argon concentration, the improvement of the heat transfer coefficient of coated glass with inflation is more obvious than that of ordinary insulating glass.
Currently, in the architectural glass industry, it is generally believed that the initial inert gas content of inflatable insulating glass should not be less than 90%, taking into account the effect of inflating on improving the insulation performance of insulating glass and the service life of insulating glass.
2. The quality evaluation method of inflatable insulating glass
Filling the middle layer of the insulating glass with inert gas helps to improve the energy-saving effect. The higher the concentration of inert gas, the better the improvement effect. Whether the inflatable insulating glass is filled with inert gas, what the gas concentration is, and what the gas retention rate is are the keys to evaluating the quality of the inflatable insulating glass. Inert gases are colorless and odorless. How to determine the quality of inflated insulating glass through relevant detection methods is the focus of research. Currently, there are three methods for analyzing and detecting inert gases: high-voltage electric spark method, oxygen paramagnetic method, and gas chromatography method.
2.1 High voltage electric spark method
2.1.1 Detection principle
The high-voltage electric spark method uses high-voltage sparks generated by the equipment to penetrate the glass and the intermediate gas layer, activating the plasma of inert gas molecules to emit radiation waves. Through emission spectroscopy, the instrument collects the photons for analysis. The thus measured spectrum is compared with data from the instrument's internal standard to determine the inert gas concentration inside the glass.
2.1.2 Method application
This method is a non-destructive testing method. It can repeatedly detect the content of argon and krypton gas in the insulating glass. It will not cause any damage to the insulating glass and will not affect the subsequent use of the glass. It is suitable for laboratories and automated insulating glass. Inflatable plate press processing production line and engineering on-site inspection. At present, the equipment is mainly a Gasglass Handheld type instrument from Finland Sparkle Co., Ltd. The instrument is easy to carry, simple, and fast to operate, see Figure 2.
Figure 2 The Gasglass Handheld type instrument from Finland Sparkle Co., Ltd.
Based on the principle of the high-voltage electric spark method, it also has certain limitations. Electric sparks cannot pass through thicker laminated glass and Low-E coating glass, so this method is helpless for products such as double low-e coating insulating glass and thicker double laminated insulating glass. Moreover, this method is more sensitive to the influence of background light, so it has higher requirements on the background environment during testing.
Currently, the high-voltage spark method is adopted by the American standard ASTM E 2649-2009 "Standard Test Method for Determining Argon Concentration in Sealed Insulated Glass Units Using Spark Emission Spectroscopy". However, based on the limitations of the testing principle and method, the standard only targets argon gas. The insulating glass with a content of not less than 70% is tested, and only the initial gas content of argon in the insulating glass is tested.
2.2 Oxygen paramagnetic methodrv
2.2.1 Detection principle
The magnetic susceptibility of oxygen is hundreds of times higher than that of ordinary gases, and the magnetic susceptibility of a mixed gas depends almost entirely on the amount of oxygen contained. This method uses the paramagnetic properties of oxygen to determine the oxygen content based on the magnetic susceptibility of the mixed gas, thereby calculating the content of inert gas in the mixed gas. The relative magnetic susceptibility of some gases is shown in Table 2.
Table 2 The relative magnetic susceptibility of some gases
| Air | O2 | NO | NO2 | N2 | CO2 | H2 | Ar | CH4 | NH3 | Water vapor |
| The relative magnetic | +100 | +43.8 | +6.2 | -0.42 | -0.61 | -0.12 | -0.59 | -0.37 | -0.57 | -0.4 |
2.2.2 Method application
This method is destructive. The test sample is the gas in the middle layer. When sampling, the outer sealant needs to be removed first, and then a syringe is used to penetrate the aluminum strip into the middle layer to extract the gas sample. The method is only suitable for quality testing in laboratories and production lines and is not suitable for engineering field testing. This method is simple and convenient to operate, but the test accuracy is slightly worse than the other two methods, and it can only measure the content of inert gas in the middle layer of insulating glass, and cannot determine the type of inert gas. This method is currently adopted by the international industry standard "Insulating Glass", which uses this method to detect the initial gas content and gas seal durability performance of insulating glass. The inert gas analyzer is calibrated before the test. The calibration uses dry air with a determined oxygen concentration and argon or krypton gas with a purity of more than 99.99%. The initial gas content is tested on three pieces of insulating glass. Insert the syringe into the middle layer of the insulating glass and push and inhale it twice. Then take a 20 mL gas sample and inject it into the equipment for testing. The initial gas concentration of the three samples should not be less than 85 %. The objects of the gas seal durability test are 3 pieces of insulating glass that have undergone accelerated durability testing. The test method is the same as the initial gas concentration. After the accelerated durability test, the inert gas content of the insulating glass shall not be less than 80%.
2.3 Gas Chromatography
2.3.1 Detection principle
Due to the differences in properties and structures of the substances being tested, the distribution coefficients of each component between the two phases are different. When the sample is brought into the chromatographic column by the carrier gas, the components are repeatedly distributed between the two phases. Although the carrier gas flow rate is the same, the adsorption or dissolution capacity of each component in the fixed phase is different, resulting in the running speed of each component on the chromatographic column. different. After a certain period of flow, they will separate from each other, thereby achieving the separation effect, and flow out of the chromatographic column into the detector in sequence. After amplification of the electronic signal, the chromatographic peaks of each component are depicted on the recorder or chromatographic data processor. Qualitative and quantitative analysis of each component of the sample can be made based on the difference in retention time and peak area of each chromatographic peak.
2.3.2 Method application
The test sensitivity and accuracy of this method are higher than the other two methods, and it can analyze nanogram-level samples, but it is only suitable for laboratory testing and is not suitable for production quality control and on-site testing. This method requires a large investment in equipment and complicated operations and requires testers with professional backgrounds to conduct the test. This method is currently adopted by the EU standard EN1279-3:2002 to test the gas concentration and gas leakage rate of insulating glass. The test of gas leakage rate requires extremely high accuracy, and the gas loss is calculated in milligrams, so only gas chromatography can meet the test requirements.
The entire process of gas leak rate testing of insulating glass is very complex. After the test sample undergoes the accelerated durability test, it is placed in a sealed system of a "ring-shaped container". The internal size of the system is only slightly larger than the insulating glass, leaving a certain amount of residual air around the glass. The entire system is placed in a constant-temperature environment. measurement environment. There is always working gas (helium) flowing in the system during the test. When the insulating glass causes the internal inert gas to leak out of the insulating glass due to the thermomechanical stress caused by the accelerated durability test, it can be captured and collected by the working gas. and transferred to the gas chromatograph for precise analysis and testing of the inert gas content.
3. Conclusion
(1) Whether using manual argon gas filling equipment or automated plate pressure filling production line, Insulating glass filled with inert gas has attracted more and more attention as an emerging product for building energy conservation and environmental protection. At present, argon has become the most widely used inert gas due to its high-cost performance. Filling the insulating glass with a certain content of argon gas can improve the thermal insulation and sound insulation properties of the insulating glass.
(2) Among the three current testing methods for testing the performance of inert gases in insulating glass, gas chromatography has the highest testing accuracy and can meet the testing requirements for gas leakage rate, but it is only suitable for laboratory testing; high-voltage spark method equipment It is easy to operate and can meet the requirements of production quality control and engineering field testing. It can perform non-destructive testing, but its scope of application has certain limitations and cannot meet the testing needs of high-end products such as double-coated hollows and double-layered hollows, as well as gas leakage rates; The test process of this oxygen paramagnetism method is relatively simple and suitable for production quality control and laboratory testing, but the test accuracy is low and it cannot characterize the type of inert gas.
(3) At present, the application of inflatable insulating glass in some developing countries is still in its infancy, and there are no published relevant product standards and method standards that require the performance of inflated insulating glass. Developed countries in Europe and the United States have formulated relevant standards for gas chromatography. For example, the American standard ASTM E2269-2005 and the European standard EN 1279-3-2002 respectively stipulate the gas chromatography method for inert gas concentration and inert gas leak rate detection. The gas leakage rate is one of the most important properties of inflatable insulating glass. Only gas chromatography can meet its testing requirements. Therefore, LIJIANG Glass believes that the establishment of a gas chromatography method for inert gas analysis will play a very positive role in the promotion and application of inflatable insulating glass.
For more information about insulating glass processing equipment and insulating glass processing accessories, please click here to learn more.